
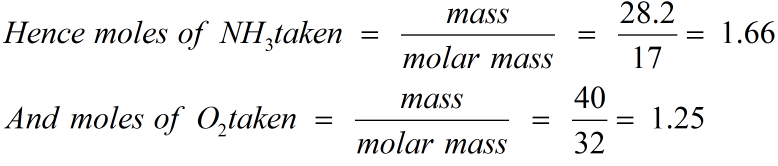

Hence, molecular formula is same as empirical formula, viz.,Fe 20 3. Given that the molar mass of the oxide is 159.8 g mol -1(Atomic mass: Fe = 55.85, O = 16.00 amu) Calculation of Empirical Formula. Determine the molecular formula of an oxide of iron in which the mass percent of iron and oxygen are 69.9 and 30.1 respectively. Thus, Cu that can be obtained from 159.5 g of CuS0 4 = 63.5 g How much copper can be obtained from 100 g of copper sulphate ( CuSO 4 ) ? (Atomic mass of Cu= 63.5 amu)Īnswer: 1 mole of CuS0 4 contains 1 mole (1 g atom) of Cu Molar mass of nitric acid HNO 3 = 1 + 14 + 48 = 63 gmol -1 Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g mL -1 and the mass percent of nitric acid in it is being 69%.Īnswer: Mass percent of 69% means that 100 g of nitric acid solution contain 69 g of nitric acid by mass. Molar mass of sodium acetate is 82.0245 g mol -1Īnswer: 0.375 M aqueous solution means that 1000 mL of the solution contain sodium acetate = 0.375 mole Calculate the mass of sodium acetate ( CH 3 COONa) required to make 500 mL of 0.375 molar aqueous solution. 16 g of dioxygen can combine only with 0.5 mole of carbon.C0 2 produced again is equal to 22 g. (iii) Here again, dioxygen is the limiting reactant. (ii) As only 16 g of dioxygen is available, it can combine only with 0.5 mole of carbon, i.e., dioxygen is the limiting reactant. Therefore,C0 2 produced from the combustion of 1 mole of carbon = 44 g. (iii) 2 moles of carbon are burnt in 16 g of dioxygen.Īnswer: The balanced equation for the combustion of carbon in dioxygen/air is
(ii) 1 mole of carbon is burnt in 16 g of dioxygen.

Calculate the amount of carbon dioxide that could be produced when Determine the empirical formula of an oxide of Iron which has 69.9 % iron and 30.1 % dioxygen by mass.
MOLAR MASS OF NH3 MANUAL
NCERT Solutions Class 11 Chemistry Chemistry Lab Manual Chemistry Sample Papers Stoichiometry and Stoichiometric Calculations Properties of Matter and their Measurement Topics and Subtopics in NCERT Solutions for Class 11 Chemistry Chapter 1 Some basic Concepts of Chemistry : Section Name NCERT Solutions for Class 11 Chemistry Chapter 1 Some basic Concepts of Chemistry Here we have given NCERT Solutions for Class 11 Chemistry Chapter 1 Some basic Concepts of Chemistry. NCERT Solutions for Class 11 Chemistry Chapter 1 Some basic Concepts of Chemistry are part of Class 11 Chemistry NCERT Solutions. Lakhmir Singh Science Class 8 Solutions.PS Verma and VK Agarwal Biology Class 9 Solutions.NCERT Solutions for Class 9 Foundation of IT.NCERT Solutions for Class 9 Social Science.NCERT Solutions for Class 10 Foundation of Information Technology.NCERT Solutions For Class 10 Hindi Kritika.NCERT Solutions For Class 10 Hindi Kshitiz.NCERT Solutions For Class 10 Hindi Sparsh.NCERT Solutions For Class 10 Hindi Sanchayan.NCERT Solutions for Class 10 Social Science.NCERT Solutions for Class 11 Indian Economic Development.NCERT Solutions for Class 11 Political Science.NCERT Solutions for Class 11 Psychology.NCERT Solutions for Class 11 Entrepreneurship.NCERT Solutions for Class 11 Accountancy.NCERT Solutions for Class 11 Business Studies.NCERT Solutions for Class 11 Computer Science (Python).NCERT Solutions for Class 12 Psychology.NCERT Solutions for Class 12 Political Science.NCERT Solutions for Class 12 Entrepreneurship.NCERT Solutions for Class 12 Macro Economics.NCERT Solutions for Class 12 Micro Economics.NCERT Solutions for Class 12 Accountancy.NCERT Solutions for Class 12 Business Studies.NCERT Solutions for Class 12 Computer Science (C++).NCERT Solutions for Class 12 Computer Science (Python).
MOLAR MASS OF NH3 PDF


 0 kommentar(er)
0 kommentar(er)
